Denosumab is a human monoclonal antibody used to treat osteoporosis (Prolia®) and metastatic bone disease (Xgeva®). Denosumab’s mechanism of action is at the surface receptors on preosteoclasts called RANK (Receptor Activation of Nuclear Kappa-B). Denosumab is a RANK inhibitor, which decreases the maturation of osteoclasts.
This ultimately protects the bone from resorbing and counters the progression of bone disease. In contrast to Bisphosphonates (half-life = ten years), Denosumab has a very short half-life (25–32 days) and does not incorporate into bone; therefore, bone cellular remodeling recovers rapidly after drug cessation.
Both Denosumab and Bisphosphonates have been linked to Medication-related osteonecrosis of the jaw (MRONJ). Although there exist several oral triggers for developing MRONJ, invasive surgical procedures (e.g., extractions, implant placement, bone grafting) appear to place patients at a higher risk.
Patients on Xgeva are usually on a monthly regimen with a higher dose of Denosumab. Because of this, any invasive surgeries are classified as an ABSOLUTE CONTRAINDICATION. However, there exists a great debate on how dentists should treat patients on Prolia, which is administered every six months. Most commonly, three treatment options have been used for many years with no general consensus or literature supporting a particular ideal treatment regimen.
- No Treatment: There exists one school of thought which classifies any type of dental surgery with patients on Prolia as an ABSOLUTE CONTRAINDICATION. However, there exists little to no literature that supports this position.
- No Modification of Prolia: Another school of thought is to not modify the medication in any way and treat the patient accordingly.
Potential Risk: Many case studies have reported an association between invasive dental surgery and the development of MRONJ with patients on Prolia. Although the reported incidence is very low, the resultant debilitating effects can be significant. In addition, even with informed consent, clinicians may place themselves in a possible medical-legal issue if a significant adverse event results.
- Drug Holiday / Drug Cessation of Prolia: The cessation of Prolia has become a very popular treatment protocol. The patient is informed that the administration of Prolia will be delayed for a period of time until the surgical procedure and complete healing are complete.
Potential Risk: Studies have shown that the treatment interruption of Densumab leads to the reversal of the bone mineral density effect to pretherapy levels within one year of discontinuation. 1 In addition, it has been reported that for patients at an increased risk of fragility fractures, drug cessation has been associated with a high risk of rebound vertebral fractures.
Therefore, many physicians are reluctant to approve a discontinuation of the drug.2 Recently (2020), a 4th option has been recommended.
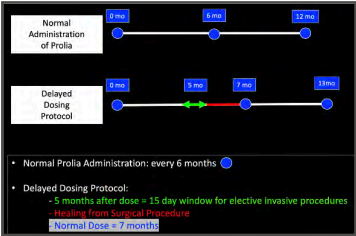
- Delayed Dosing Window: Campisi et. al in two papers has recommended a “delayed dosing window” which utilizes the pharmakinetics of Denosumab in identifying a safe “time interval” for elective invasive procedures without restrictions.3
- It has been postulated that there exists a window of two months in which the possibility of developing MRONJ is very low. This window starts five months after a Prolia dose and ends at the 7th month. It has been shown that over this 2-month time span, bone remodeling and soft-tissue healing are not altered, which allows for normal healing processes. 4
Potential Risk: A one-month postponement of Prolia has been shown not to compromise bone mineral density, and the fracture risk remains very low. Therefore, there are no significant risks with this protocol.
DELAYED DOSING PROTOCOL 5
- Surgery Date: determine the 5th month after the last administration of Prolia. Schedule the procedure within a 15-day window after the 5th month.
- Medical Clearance: consult directly with the patient’s physician concerning the delayed dosing window. The physician should contact the patient to modify the administration date.
- Prophylactic Antibiotics: make sure the patient is treated with pre- and post-op prophylactic antibiotics (Beta-Lactam)
- Surgical Procedure: minimize the invasiveness of the proposed surgery and reduce surgical duration.
1 Altay, M. R. (2017). Observations following discontinuation of long-term denosumab therapy. Osteoporos Int, vol. 28 (5), 1723- 1732. .
2 Athanasios D (2017). Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J Bone Miner Res, vol. 32 (6), 1291-1296.
3 Campisi, Giuseppina, et al. “Simplifying the dental/periodontal management of patients with metabolic bone fragility receiving treatment with denosumab.” Qeios (2020).
4 Campisi, Giuseppina, et al. “MedicationRelated Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020.” International Journal of Environmental Research and Public Health 17.16 (2020): 5998.
5 Olga Di Fede, (2018). The Dental Management of Patients at Risk of MedicationRelated Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. BioMed Research International, vol. 2018, 1-10.